900 319 0030
enquiry@shankarias.in
Why in news?
Chemistry Nobel 2021 was awarded to German scientist Benjamin List and Scotland-born scientist David W.C. MacMillan who independently developed a new way of catalysis – asymmetric organocatalysis in 2000.
What are catalysts?
What is asymmetric organocatalysis?
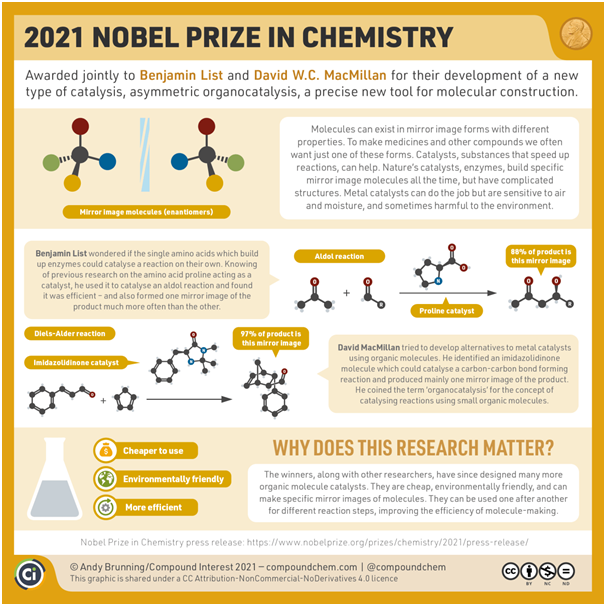
Why is the discovery so significant?
Source: The Hindu, The Indian Express