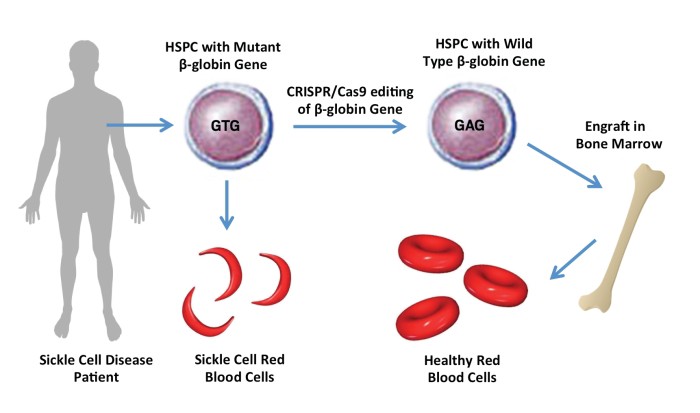
The first therapy based on gene editing technology Crispr-Cas9 for sickle cell disease and thalassaemia has been approved in UK.
|
About |
Sickle Cell Anaemia |
Thalassemia |
|
Disease |
An inherited blood disorder where people who inherit a pair of genes from both parents experience symptoms like severe anaemia. |
|
|
Effect on haemoglobin chain |
Caused by a mutation in the haemoglobin-β gene found on Chromosome 11 affecting only the beta chain |
Production of either the alpha or beta chains is reduced resulting in either alpha-thalassemia or beta-thalassemia |
|
Haemoglobin production |
Mutation in haemoglobin chains makes them into a crescent shape under low oxygen level |
Caused by reduced production of haemoglobin chains |
|
Effects |
Pain, fever, infection, stroke and organ damage |
Fatigue, shortness of breath, irregular heartbeats and need blood transfusions throughout their life |
|
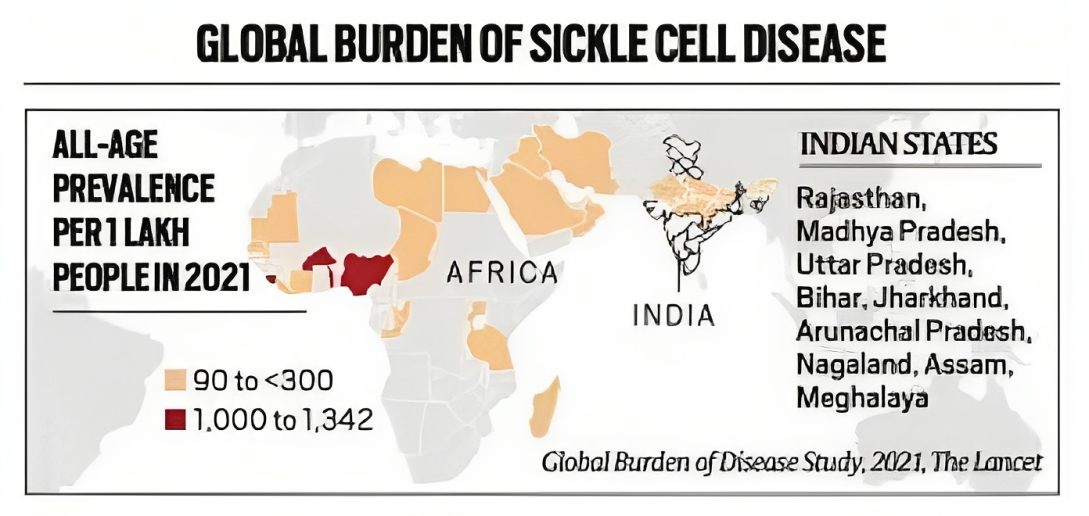
Status in India |
An estimated 30,000-40,000 children in India are born with the disorder every year. |
India has the largest number of children with thalassaemia (about 1-1.5 lakh). |
|
|
|
|
|
Treatment |
Treated by blood transfusions, iron supplements, or stem cell transplants. |
|
|
Significance |
Challenges |
|
Efficacy- It restores haemoglobin production and alleviates symptoms in most patients. |
Limited authorization- It is currently approved in the UK only and is being reviewed by other regulatory bodies. |
|
Pain reliever- It reduces the need for blood transfusions and pain crises in the patients. |
Health inequity- It is expensive, thereby limiting the accessibility in poor countries. |
|
Reliable- No serious safety concerns were reported, but long-term effects are still being monitored. |
Inaccuracy- There are concerns with potential off targets effects of CRISPR editing, which could cause unwanted changes in other parts of the genome. |
References